Abstract
Background: Patients with diffuse large B-cell lymphoma (DLBCL) in the elderly face challenges in treatment due to comorbidities and poor tolerance of chemotherapy. Age >60 years is an unfavorable risk factor in DLBCL in the International Prognostic Index (IPI) system. Besides, increased proportion of ABC/non-GCB subgroups, complex molecular features also lead to unfavorable prognosis with distinct biological or immune microenvironment features in elderly patients. Although clinical benefit of single-agent anti-PD-1 antibody in DLBCL was not obvious, pre-use anti-PD-1 antibody as first-line may be an opportune for elderly DLBCL. Toripalimab, a recombinant, humanized programmed death receptor-1 (PD-1) monoclonal antibody has been approved by the NMPA for the treatment of various cancers.
Methods: Patients aged 60-85 years, ECOG 0-2 with untreated DLBCL or grade 3b follicular lymphoma and at least one measurable or evaluable lesion were enrolled. This study consisted of a phase 1b (Toripalimab dose escalation) followed by a Phase 2 expansion portion. In the phase Ib, a standard "3+3" design was utilized to identify the MTD, DLT and recommended phase 2 dosage (RP2D) of Toripalimab.In phase 2, patients received 2 cycles of Toripalimab (RP2D) plus Rituximab (375mg/m2) every 3 weeks. They allowed to accept two additional cycles of Toripalimab plus Rituximab (To-Ri) if they obtained complete response (CR) after the first 2 cycles. To-Ri followed by R-CHOP (21) up to 6 cycles without Toripalimab then Toripalimab (RP2D) maintenance 6 cycles (q 30 days) after CR or CMR post R-CHOP. All cases more than 75-year-old received R-miniCHOP. The primary endpoints were objective response rate (ORR) based on Lugano 2016 criteria and clinical benefit rate. Secondary endpoints were progression-free survival (PFS), overall survival (OS) and safety. Adverse events (AEs) were defined according to CTCAE 5.0. This trial was registered at ClinicalTrials.gov (NCT04058470).
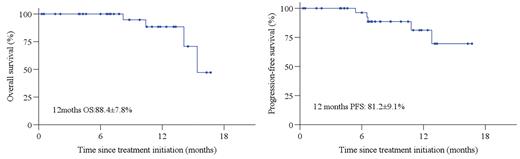
Results: From December 2020 to July 2022, 37 eligible patients were enrolled. Median age 67 years (range, 60-83), 16 (43.2%) male, 20 (54.1%) patients with stage III/IV, 27(73.3%) patients with extranodal disease, 8 (21.6%) patients with IPI score ≥ 3 points, and non-GCB(n=25), double expression (DEL, n=7), EBV+DLBCL(n=5), double/triple hit lymphoma (DHL/THL, n=2). In phase Ib, no MTD or DLT events were observed, RP2D of Toripalimab was 240mg. Of 34 response evaluable patients, 14 patients completed To-Ri×4, 26 (59.1%) achieved response including 15 (44.1%) patients with CR post To-Ri treatment. Key responded to To-Ri including non-GCB (81.8%, n=18), GCB (58.3%, n=7), EBV+DLBCL (50%, n=2), DEL (87.5%, n=7), DHL/THL (100%, n=2), primary gastrointestinal tract involved (75%, 6/8) and primary nasal involved (87.5%, 7/8). Five patients are still under treatment, ORR and CR post R-CHOP were 100% (32/32) and 87.5% (28/32). With a median follow-up of 8.2 months, estimated 1-year PFS and were 81.2% and 88.4%. Thirty-six (97.3%) patients reported treatment-related AEs (TRAEs). The most frequently observed (≥10%) TRAEs were abnormal liver function (43.2%), anemia (43.2%), lymphopenia (32.4%), neutropenia (27.0%), hypothyroidism (21.6%), nausea (18.5%). This clinical trial is still on going.
Conclusion: Toripalimab plus Rituximab as a first-line treatment then followed by R-CHOP yielded high CR rate and manageable toxicities in elderly with newly diagnosed DLBCL, especially for patients with extranodal disease and high grade lymphoma. This therapeutic strategy deserves further clinical investigation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal